Qinlock demonstrated powerful PFS results2
Qinlock provided superior median PFS in the primary analysis2
Primary endpoint: PFS
- 6.3 months vs 1.0 month (HR=0.15 [95% CI, 0.09‑0.25]; P<0.0001)2
Qinlock demonstrated consistent PFS results at long‑term follow‑up3*
long-term follow-up analysis
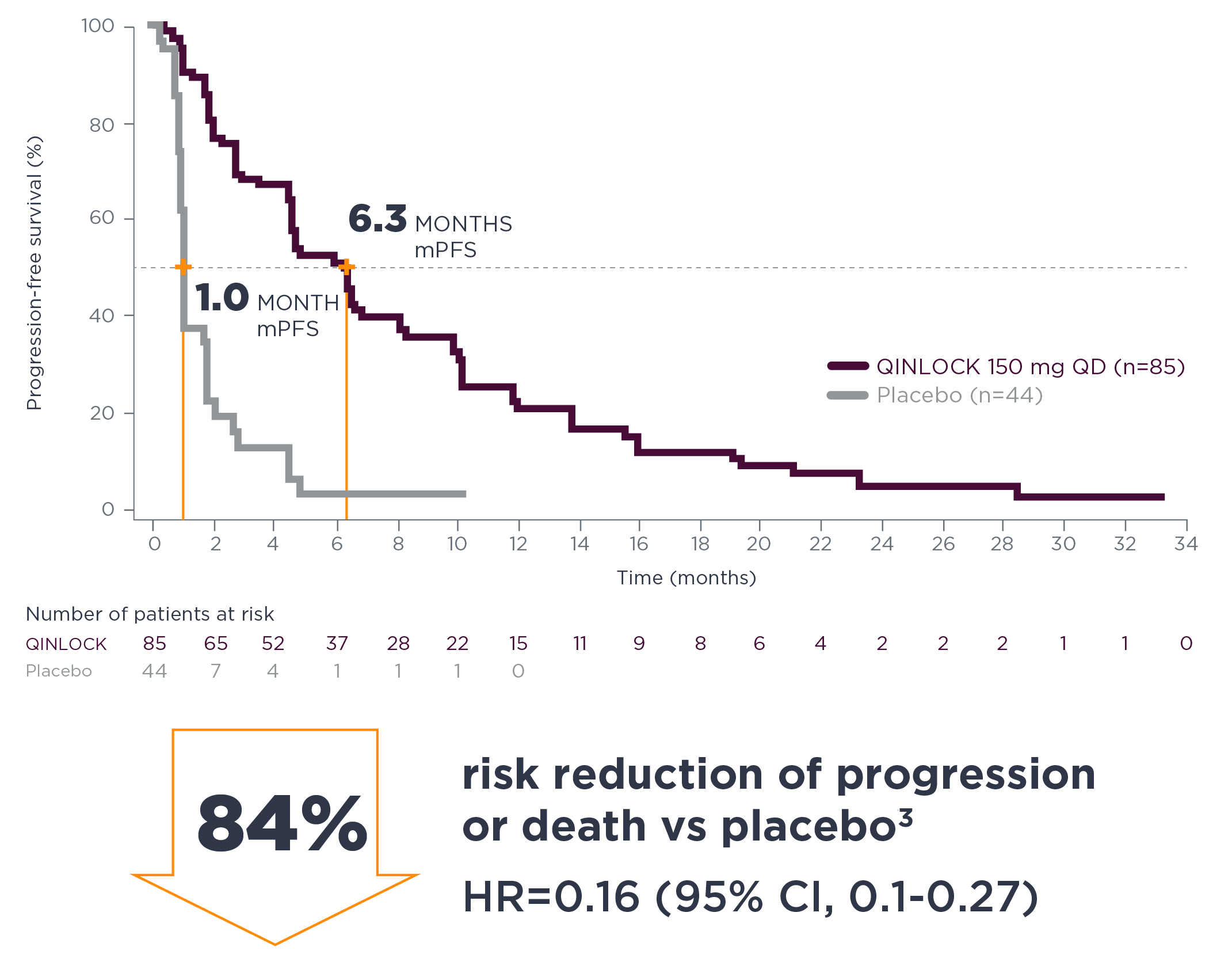
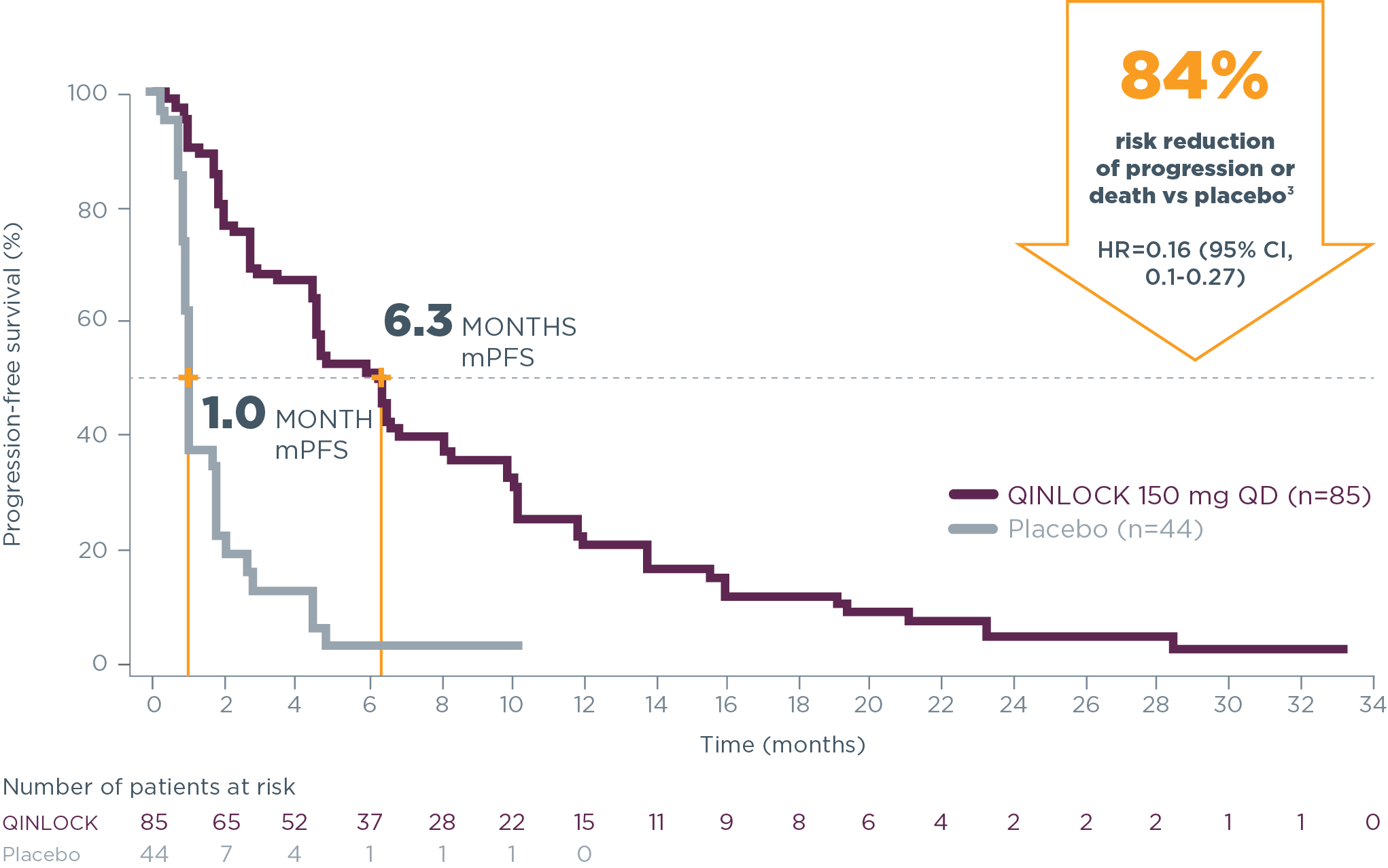
*The follow-up analysis was conducted approximately 19 months from the data cutoff date in the primary analysis and was not powered to show statistical significance.3
Learn more about the latest PFS data from a Qinlock representative.
Estimated PFS in Invictus at long‑term follow‑up3
| Estimated landmark PFS | Qinlock | Placebo |
|---|---|---|
| (n=85) | (n=44) | |
| 6-months PFS (95% CI) | 51.0% (39.4–61.4) | 3.2% (0.2–13.8) |
| 12-months PFS (95% CI) | 22.2% (13.4–32.4) | NE (NE–NE) |
| 18-months PFS (95% CI) | 11.8% (5.6–20.6) | NE (NE–NE) |
NE=not estimable.
Qinlock provided generally consistent PFS results across primary mutations and other patient subgroups3,4
PFS results for Qinlock vs placebo in select patient subgroups at long‑term follow‑up3,4†
Long‑term follow‑up analysis
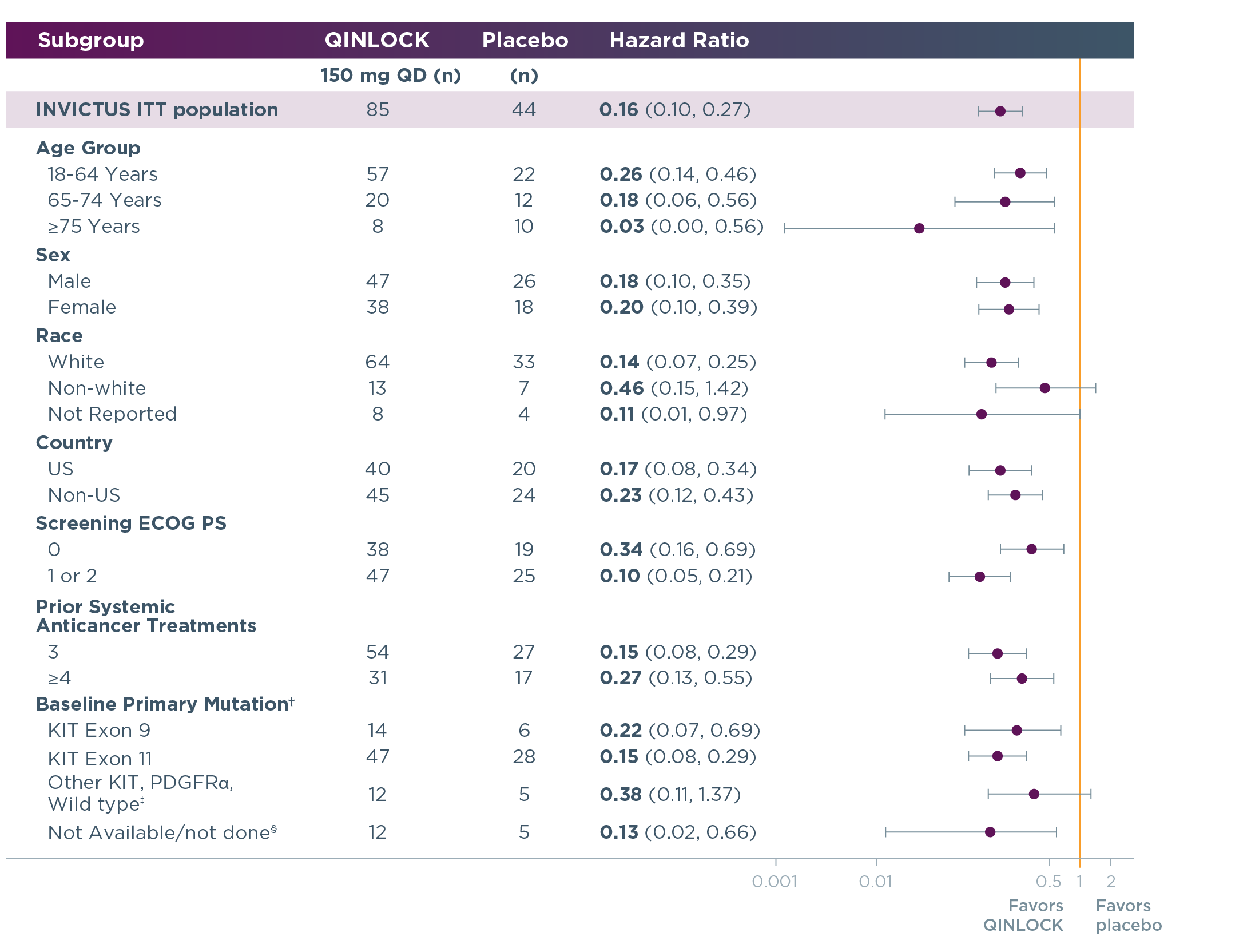
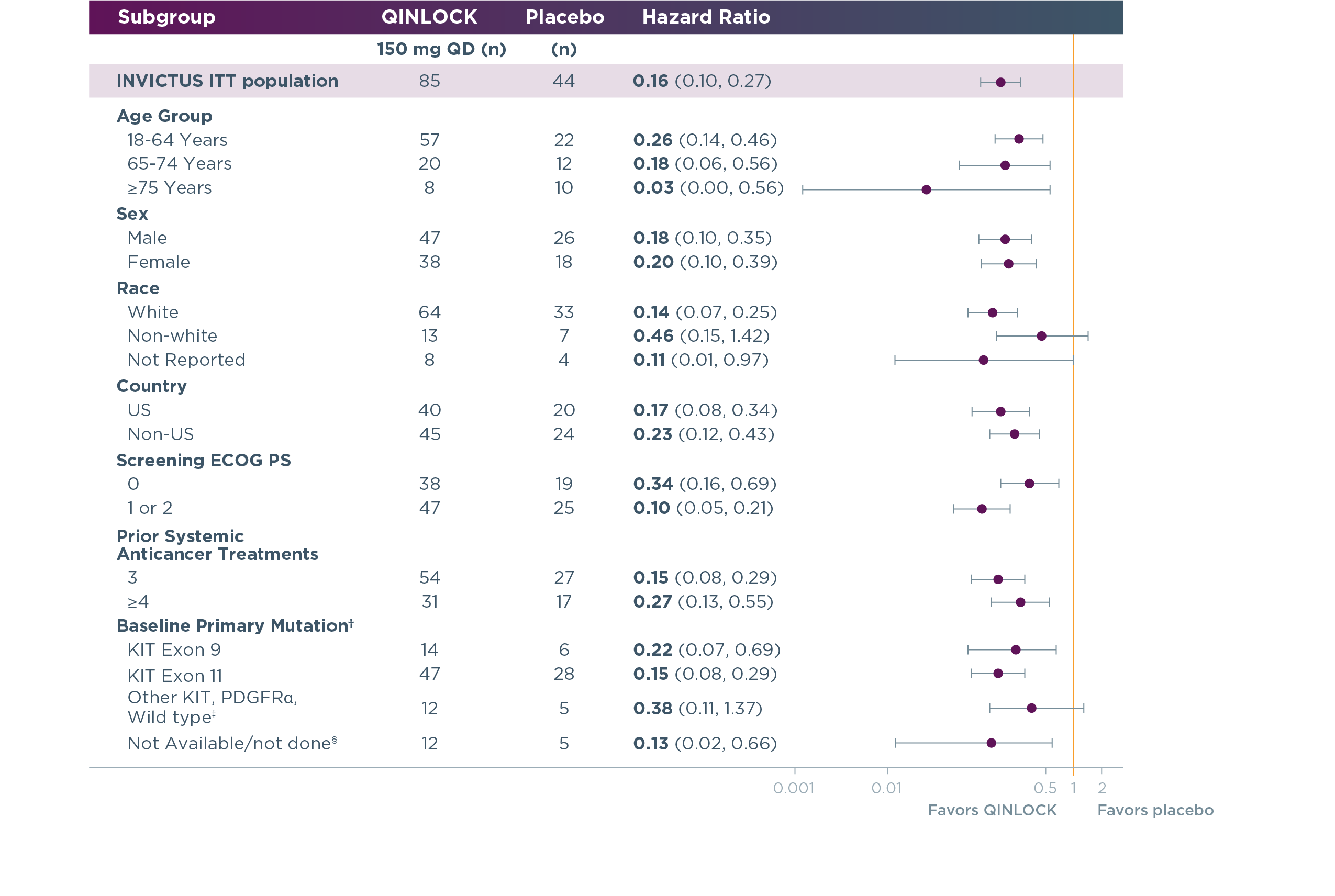
†This analysis was exploratory in nature; it did not control for type 1 error and was not powered to determine treatment effect in any subgroup. Hazard ratio for PFS based on baseline primary mutation status was retrospectively assessed after 9 months of additional follow-up (data cut-off: March 9, 2020) following the primary analysis (data cutoff: May 31, 2019) in tumor samples by tumor biopsy from evaluable patients treated with QINLOCK (n=73) and placebo (n=39).4
‡Includes other KIT exon mutations, PDGFRα mutations, and KIT/PDGFRα wild-type patients.
§Includes patients who failed sequencing due to low tumor content and patients with no specimen.
Clinically meaningful improvement in objective response rate (ORR) by BICR
Key Secondary Endpoint: ORR
Primary Analysis
9.4% Qinlock vs 0.0% Placebo
(P=0.0504)2,5‖
Long-Term Follow-up Analysis
11.8% Qinlock vs 0.0% Placebo3¶
- Median duration of response was 14.5 months with Qinlock vs NE with placebo3
‖All responses were partial responses.
¶The long-term follow-up analysis, conducted 19 months after the primary analysis, was not powered to show statistical significance.
Qinlock was associated with clinically meaningful overall survival2
Qinlock overall survival (OS) vs placebo in the primary analysis2,5#
SECONDARY ENDPOINT: OS
- 15.1 months vs 6.6 months (HR=0.36 [95% CI, 0.21‑0.62])2,5#
Qinlock demonstrated a median OS of 18.2 months at long‑term follow‑up3**
Long-term Follow-up Analysis
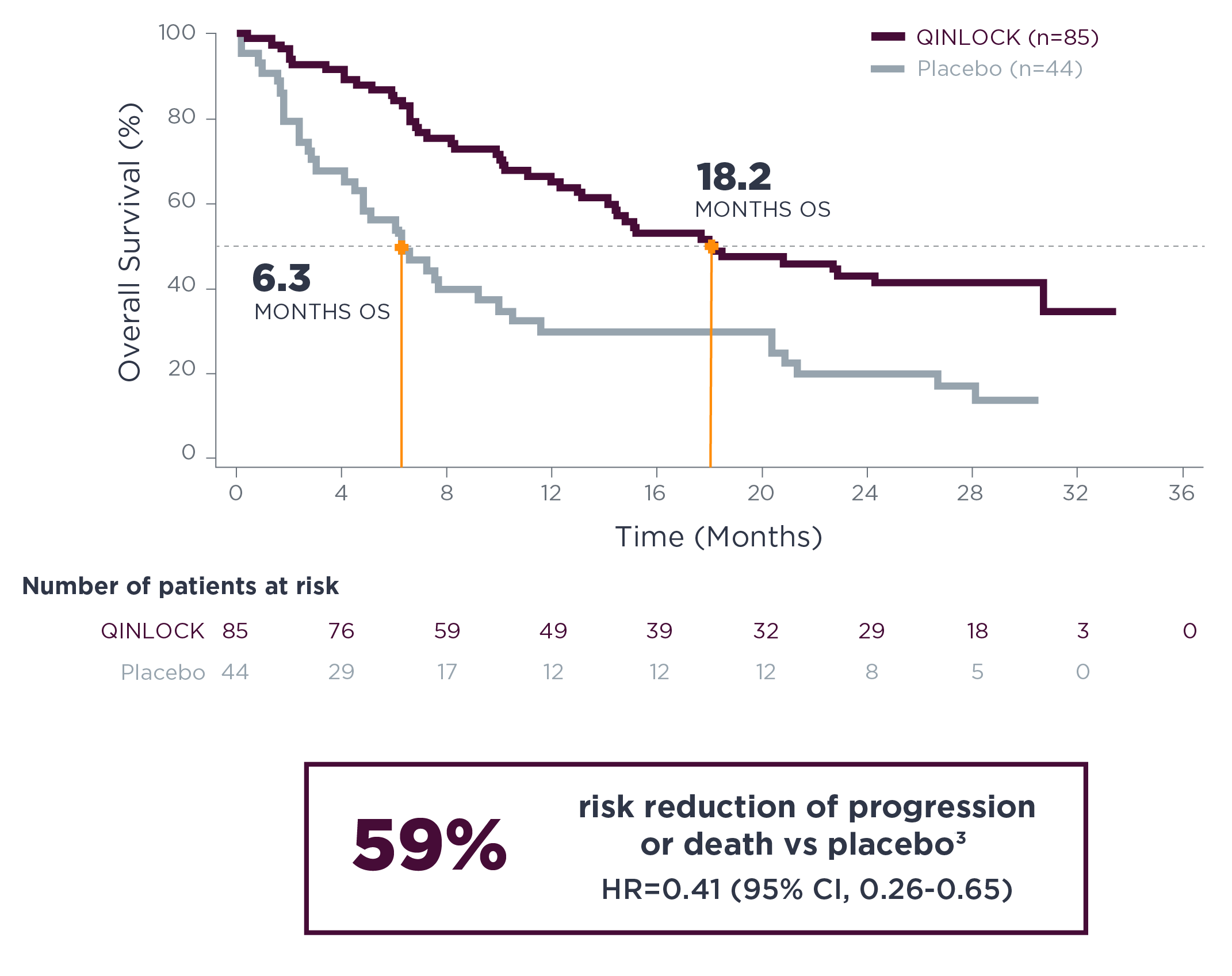
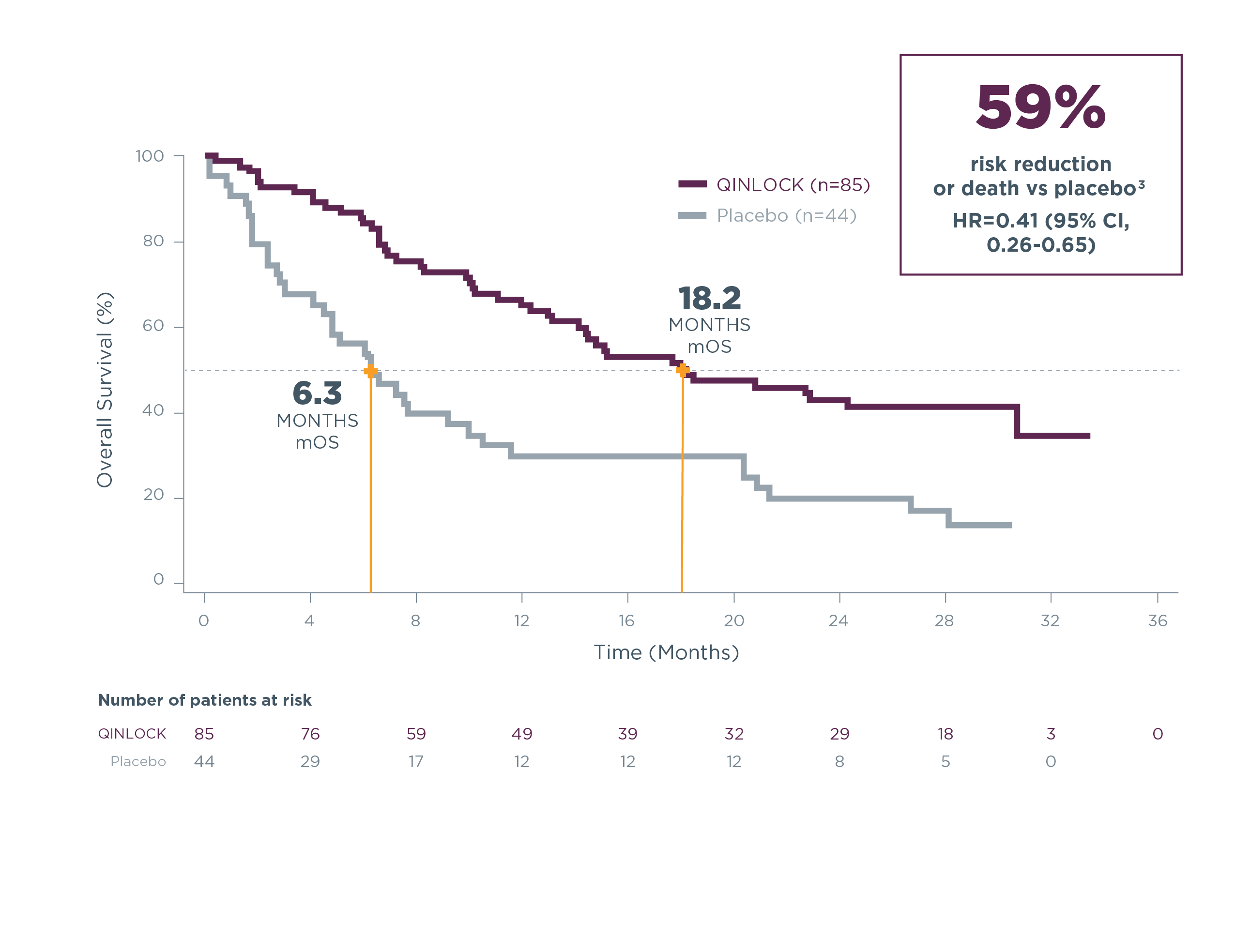
mOS=median overall survival.
OS data include all time periods. Placebo curve includes patients who crossed over to Qinlock treatment.
#OS was a secondary endpoint in the INVICTUS trial. OS was not evaluated for statistical significance as a result of the sequential testing procedure used for the secondary endpoints of ORR and OS.2,5
**The long-term follow-up analysis was conducted approximately 19 months from the data cutoff date in the primary analysis and was not powered to show statistical significance.3
Estimated OS at long-term follow-up3
| Estimated landmark OS | Qinlock | Placebo |
|---|---|---|
| (n=85) | (n=44) | |
| 6-months OS (95% CI) | 84.3% (74.5–90.6) | 55.9% (39.9–69.2) |
| 12-months OS (95% CI) | 65.1% (53.6–74.5) | 29.7% (16.8–43.7) |
| 18-months OS (95% CI) | 50.1% (38.5–60.7) | 29.7% (16.8–43.7) |
| 24-months OS (95% CI) | 42.8% (31.5–53.7) | 19.8% (9.4–33.0) |

The Qinlock safety profile was established across a broad range of patients in the Invictus trial2,5
Rates of discontinuation and dose modification due to adverse reactions were similar between Qinlock and placebo2,7
See How Qinlock Works
Qinlock is the first and only switch-control kinase inhibitor2,8
Learn About The MOAOnce-daily Dosing
Qinlock is dosed orally, once daily, with or without food2
Dosing & AdministrationCI=confidence interval; ECOG PS=Eastern Cooperative Oncology Group Performance Status; HR=hazard ratio; ITT=intent‑to‑treat; mPFS=median progression‑free survival; mOS=median overall survival; PFS=progression‑free survival.
References: 1. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastrointestinal Stromal Tumors (GIST) V.1.2023. ©National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed November 6, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 2. Qinlock [package insert]. Waltham, MA: Deciphera Pharmaceuticals, Inc; 2023. 3. von Mehren M, Heinrich M, George S, et al. Ripretinib as ≥4th‑line treatment in patients with advanced gastrointestinal stromal tumour (GIST): Long-term update from the phase 3 INVICTUS study. Poster presented at: 2021 European Society for Medical Oncology Virtual Meeting; September 16‑21, 2021. 4. Schöffski P, Bauer S, Heinrich M, et al. Ripretinib demonstrated activity across all KIT/PDGFRA mutations in patients with fourth-line advanced gastrointestinal stromal tumor: Analysis from the phase 3 INVICTUS study. Poster presentation at: 2020 Connective Tissue Oncology Society Virtual Meeting; November 18‑21, 2020. 5. Blay JY, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(7):923-934. 6. Blay JY, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(7)(suppl):1-5. 7. von Mehren M, Attia S, Bauer S, et al. INVICTUS: A phase 3, interventional, double-blind, placebo-controlled study to assess the safety and efficacy of ripretinib as ≥4th line therapy in patients with advanced gastrointestinal stromal tumors (GIST) who have received treatment with prior anticancer therapies (NCT03353753). Oral presentation at: European Society for Medical Oncology Annual Meeting; October, 2019; Barcelona, Spain. 8. Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC‑2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug‑resistant KIT and PDGFRA variants. Cancer Cell. 2019;35(5):738‑751.
Indication & Important Safety Info
Indication
Qinlock is a kinase inhibitor indicated for the treatment of adult patients with advanced gastrointestinal stromal tumor (GIST) who have received prior treatment with 3 or more kinase inhibitors, including imatinib.
Important Safety Information
There are no contraindications for Qinlock.
Palmar‑plantar erythrodysesthesia syndrome (PPES): In INVICTUS, Grade 1–2 PPES occurred in 21% of the 85 patients who received Qinlock. PPES led to dose discontinuation in 1.2% of patients, dose interruption in 2.4% of patients, and dose reduction in 1.2% of patients. Based on severity, withhold Qinlock and then resume at same or reduced dose.
New Primary Cutaneous Malignancies: In INVICTUS, cutaneous squamous cell carcinoma (cuSCC) occurred in 4.7% of the 85 patients who received Qinlock with a median time to event of 4.6 months (range 3.8 to 6 months). In the pooled safety population, cuSCC and keratoacanthoma occurred in 7% and 1.9% of patients, respectively. In INVICTUS, melanoma occurred in 2.4% of the 85 patients who received Qinlock. In the pooled safety population, melanoma occurred in 0.9% of patients. Perform dermatologic evaluations when initiating Qinlock and routinely during treatment. Manage suspicious skin lesions with excision and dermatopathologic evaluation. Continue Qinlock at the same dose.
Hypertension: In INVICTUS, Grade 1–3 hypertension occurred in 14% of the 85 patients who received Qinlock, including Grade 3 hypertension in 7% of patients. Do not initiate Qinlock in patients with uncontrolled hypertension. Monitor blood pressure as clinically indicated. Based on severity, withhold Qinlock and then resume at same or reduced dose or permanently discontinue.
Cardiac Dysfunction: In INVICTUS, cardiac failure occurred in 1.2% of the 85 patients who received Qinlock. In the pooled safety population, cardiac dysfunction (including cardiac failure, acute left ventricular failure, diastolic dysfunction, and ventricular hypertrophy) occurred in 1.7% of patients, including Grade 3 adverse reactions in 1.1% of patients.
In INVICTUS, Grade 3 decreased ejection fraction occurred in 2.6% of the 77 patients who received Qinlock and who had a baseline and at least one post‑baseline echocardiogram. Grade 3 decreased ejection fraction occurred in 3.4% of the 263 patients who received Qinlock and who had a baseline and at least one post‑baseline echocardiogram.
In INVICTUS, cardiac dysfunction led to dose discontinuation in 1.2% of the 85 patients who received Qinlock. The safety of Qinlock has not been assessed in patients with a baseline ejection fraction below 50%. Assess ejection fraction by echocardiogram or MUGA scan prior to initiating Qinlock and during treatment, as clinically indicated. Permanently discontinue Qinlock for Grade 3 or 4 left ventricular systolic dysfunction.
Risk of Impaired Wound Healing: Qinlock has the potential to adversely affect wound healing. Withhold Qinlock for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of Qinlock after resolution of wound healing complications has not been established.
Photosensitivity: Qinlock may cause photosensitivity reactions. In 621 patients treated with Qinlock in clinical trials, photosensitivity reactions occurred in 0.6% of patients. Advise patients to limit direct ultraviolet exposure during treatment with Qinlock and for at least 1 week after discontinuation of treatment.
Embryo‑Fetal Toxicity: Qinlock can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment and for 1 week after the last dose. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment and for 1 week after the last dose. Qinlock may impair fertility in males of reproductive potential.
Adverse Reactions: The most common adverse reactions (≥20%) were alopecia, fatigue, nausea, abdominal pain, constipation, myalgia, diarrhea, decreased appetite, PPES, and vomiting. The most common Grade 3 or 4 laboratory abnormalities (≥4%) were increased lipase and decreased phosphate.
The safety and effectiveness of Qinlock in pediatric patients have not been established.
Administer strong CYP3A inhibitors with caution. Monitor patients who are administered strong CYP3A inhibitors more frequently for adverse reactions. Avoid concomitant use with strong and moderate CYP3A inducers. If a moderate CYP3A inducer cannot be avoided, increase Qinlock dosing frequency from the recommended dose of 150 mg once daily to 150 mg twice daily during the co‑administration period. If the concomitant moderate CYP3A inducer is discontinued, resume Qinlock dosage back to 150 mg once daily 14 days after the discontinuation of the moderate CYP3A inducer.
Please see full Prescribing Information, including Patient Information.
To report SUSPECTED ADVERSE REACTIONS, contact Deciphera Pharmaceuticals, LLC, at 1-888-724-3274 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Indication & Important Safety Info
Indication
Qinlock is a kinase inhibitor indicated for the treatment of adult patients with advanced gastrointestinal stromal tumor (GIST) who have received prior treatment with 3 or more kinase inhibitors, including imatinib.
Important Safety Information
There are no contraindications for Qinlock.
Palmar‑plantar erythrodysesthesia syndrome (PPES): In INVICTUS, Grade 1–2 PPES occurred in 21% of the 85 patients who received Qinlock. PPES led to dose discontinuation in 1.2% of patients, dose interruption in 2.4% of patients, and dose reduction in 1.2% of patients. Based on severity, withhold Qinlock and then resume at same or reduced dose.
New Primary Cutaneous Malignancies: In INVICTUS, cutaneous squamous cell carcinoma (cuSCC) occurred in 4.7% of the 85 patients who received Qinlock with a median time to event of 4.6 months (range 3.8 to 6 months). In the pooled safety population, cuSCC and keratoacanthoma occurred in 7% and 1.9% of patients, respectively. In INVICTUS, melanoma occurred in 2.4% of the 85 patients who received Qinlock. In the pooled safety population, melanoma occurred in 0.9% of patients. Perform dermatologic evaluations when initiating Qinlock and routinely during treatment. Manage suspicious skin lesions with excision and dermatopathologic evaluation. Continue Qinlock at the same dose.
Hypertension: In INVICTUS, Grade 1–3 hypertension occurred in 14% of the 85 patients who received Qinlock, including Grade 3 hypertension in 7% of patients. Do not initiate Qinlock in patients with uncontrolled hypertension. Monitor blood pressure as clinically indicated. Based on severity, withhold Qinlock and then resume at same or reduced dose or permanently discontinue.
Cardiac Dysfunction: In INVICTUS, cardiac failure occurred in 1.2% of the 85 patients who received Qinlock. In the pooled safety population, cardiac dysfunction (including cardiac failure, acute left ventricular failure, diastolic dysfunction, and ventricular hypertrophy) occurred in 1.7% of patients, including Grade 3 adverse reactions in 1.1% of patients.
In INVICTUS, Grade 3 decreased ejection fraction occurred in 2.6% of the 77 patients who received Qinlock and who had a baseline and at least one post‑baseline echocardiogram. Grade 3 decreased ejection fraction occurred in 3.4% of the 263 patients who received Qinlock and who had a baseline and at least one post‑baseline echocardiogram.
In INVICTUS, cardiac dysfunction led to dose discontinuation in 1.2% of the 85 patients who received Qinlock. The safety of Qinlock has not been assessed in patients with a baseline ejection fraction below 50%. Assess ejection fraction by echocardiogram or MUGA scan prior to initiating Qinlock and during treatment, as clinically indicated. Permanently discontinue Qinlock for Grade 3 or 4 left ventricular systolic dysfunction.
Risk of Impaired Wound Healing: Qinlock has the potential to adversely affect wound healing. Withhold Qinlock for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of Qinlock after resolution of wound healing complications has not been established.
Photosensitivity: Qinlock may cause photosensitivity reactions. In 621 patients treated with Qinlock in clinical trials, photosensitivity reactions occurred in 0.6% of patients. Advise patients to limit direct ultraviolet exposure during treatment with Qinlock and for at least 1 week after discontinuation of treatment.
Embryo‑Fetal Toxicity: Qinlock can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment and for 1 week after the last dose. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment and for 1 week after the last dose. Qinlock may impair fertility in males of reproductive potential.
Adverse Reactions: The most common adverse reactions (≥20%) were alopecia, fatigue, nausea, abdominal pain, constipation, myalgia, diarrhea, decreased appetite, PPES, and vomiting. The most common Grade 3 or 4 laboratory abnormalities (≥4%) were increased lipase and decreased phosphate.
The safety and effectiveness of Qinlock in pediatric patients have not been established.
Administer strong CYP3A inhibitors with caution. Monitor patients who are administered strong CYP3A inhibitors more frequently for adverse reactions. Avoid concomitant use with strong and moderate CYP3A inducers. If a moderate CYP3A inducer cannot be avoided, increase Qinlock dosing frequency from the recommended dose of 150 mg once daily to 150 mg twice daily during the co‑administration period. If the concomitant moderate CYP3A inducer is discontinued, resume Qinlock dosage back to 150 mg once daily 14 days after the discontinuation of the moderate CYP3A inducer.
Please see full Prescribing Information, including Patient Information.
To report SUSPECTED ADVERSE REACTIONS, contact Deciphera Pharmaceuticals, LLC, at 1-888-724-3274 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
