
*Preferred 4th-line therapy option (Category 1) for certain patients with
unresectable or metastatic disease.1
For advanced GIST patients
treated with ≥3 prior TKIs,
including imatinib
Break through resistance
and provide powerful progression‑free survival2
-
6.3 months median PFS with Qinlock (n=85)
vs 1.0 month with placebo (n=44)2
HR=0.15 (95% CI, 0.09–0.25); P<0.0001
Choose Qinlock,
a standard of care in advanced
GIST after 3 TKIs, regardless of mutation status3‑5
- KIT
- PDGFRA
- WILD TYPE
See How Qinlock Works
Qinlock is the first and only switch-control kinase inhibitor for advanced GIST2,6
Learn About The MOAExpert Exchange Video Series
Watch experts from leading cancer centers share insights and research about QINLOCK
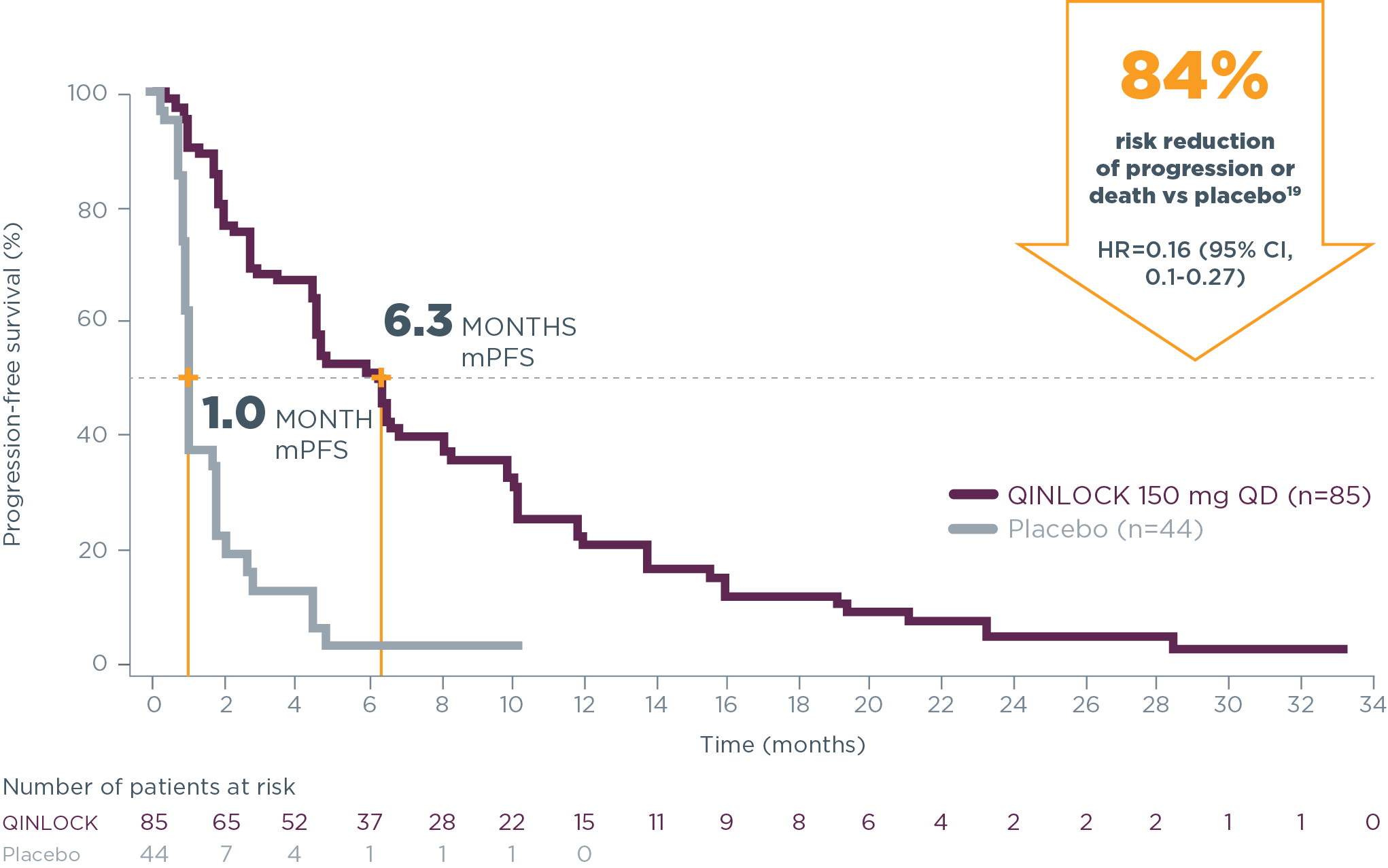
Hear From ExpertsQinlock demonstrated powerful PFS results2
Primary Endpoint: PFS
Qinlock provided superior median PFS vs placebo in the primary analysis2
- 6.3 months vs 1.0 month (HR=0.15 [95% CI, 0.09‑0.25]; P<0.0001)2
long-term follow-up analysis
Consistent PFS results at long-term follow‑up7†
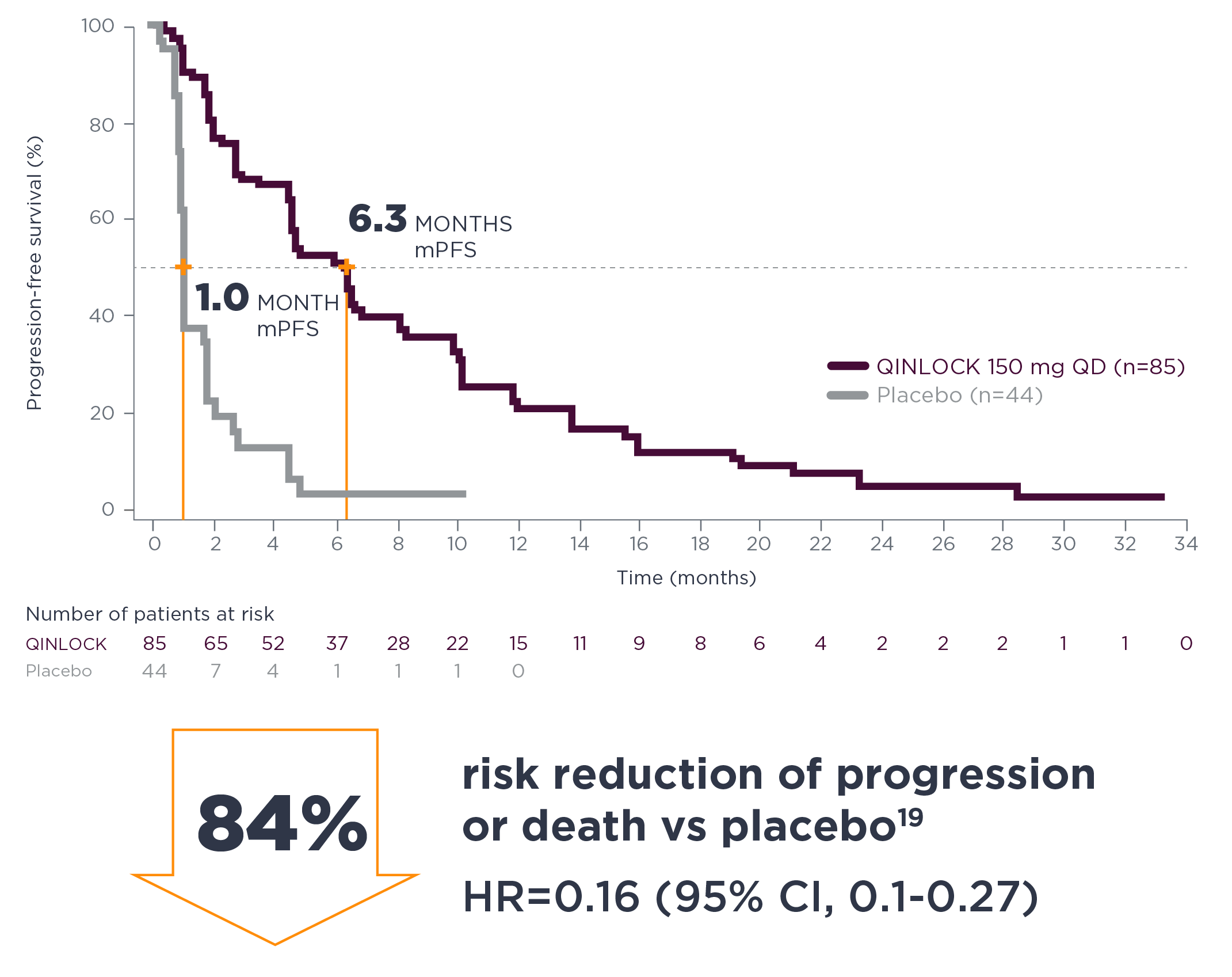
†The long-term follow-up analysis was conducted approximately 19 months from the data cutoff in the primary analysis and was not powered to show statistical significance.7
Qinlock was studied in a global, Phase 3 study in ≥4th‑line GIST2
The INVICTUS study was a global, multicenter, randomized, double‑blind, placebo‑controlled Phase 3 trial (N=129).2
The patient population of INVICTUS was the most heavily pre-treated cohort ever studied in a Phase 3, randomized, 4th‑line GIST setting.2,8
63% of patients had received 3 prior therapies
37% of patients had received ≥4 prior therapies, and some as many as 7

Explore how QINLOCK was studied
More about INVICTUS4L=4th-line; CI=confidence interval; GIST=gastrointestinal stromal tumor; HR=hazard ratio; KIT=KIT proto-oncogene receptor tyrosine kinase; NCCN=National Comprehensive Cancer Network®; PDGFRA=platelet-derived growth factor receptor alpha; PFS=progression-free survival; mPFS=median progression‑free survival; TKI=tyrosine kinase inhibitor; QD=once daily.
References: 1. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastrointestinal Stromal Tumors (GIST) V.1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed June 10, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 2. Qinlock [package insert]. Waltham, MA: Deciphera Pharmaceuticals, LLC. 3. Bauer S, Heinrich MC, George S, et al. Clinical activity of ripretinib in patients with advanced gastrointestinal stromal tumor harboring heterogeneous KIT/PDGFRA mutations in the phase III INVICTUS study. Clin Cancer Res. 2021;27(23):6333-6342. 4. Jones RL, Golčić M. Recent advances in the systemic treatment of gastrointestinal stromal tumors. Cancer Biol Med. 2023;20(10):701-705. 5. Thirasastr P, Somaiah N. Emerging data on the safety and efficacy of ripretinib for the treatment of gastrointestinal stromal tumors. Clin Exp Gastroenterol. 2023;16:11-19. 6. Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell. 2019;35(5):738-751. 7. von Mehren M, Heinrich M, George S, et al. Ripretinib as ≥4th-line treatment in patients with advanced gastrointestinal stromal tumour (GIST): Long-term update from the phase 3 INVICTUS study. Poster presented at: 2021 European Society for Medical Oncology Virtual Meeting; September 16-21, 2021. 8. Blay JY, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(7):923-934.
