
For advanced GIST patients
treated with ≥3 prior TKIs,
including imatinib
Break through resistance
Qinlock delivers what matters: powerful PFS for patients with advanced GIST2
-
6.3 months median PFS with Qinlock vs 1.0 month with placebo
HR=0.15 (95% CI, 0.09–0.25); P<0.0001
*Preferred 4th-line therapy (Category 1) for unresectable or metastatic disease.1
See How Qinlock Works
Qinlock is the first and only switch-control kinase inhibitor2,3
Learn About The MOAExpert Exchange Video Series
Watch experts from leading cancer centers share insights and the latest research about QINLOCK
Hear From ExpertsQinlock demonstrated powerful PFS results2
Qinlock provided superior median PFS vs placebo in the primary analysis2
Primary Endpoint: PFS
- 6.3 months vs 1.0 month (HR=0.15 [95% CI, 0.09‑0.25]; P<0.0001)2
Qinlock demonstrated consistent PFS results at long-term follow‑up4†
long-term follow-up analysis
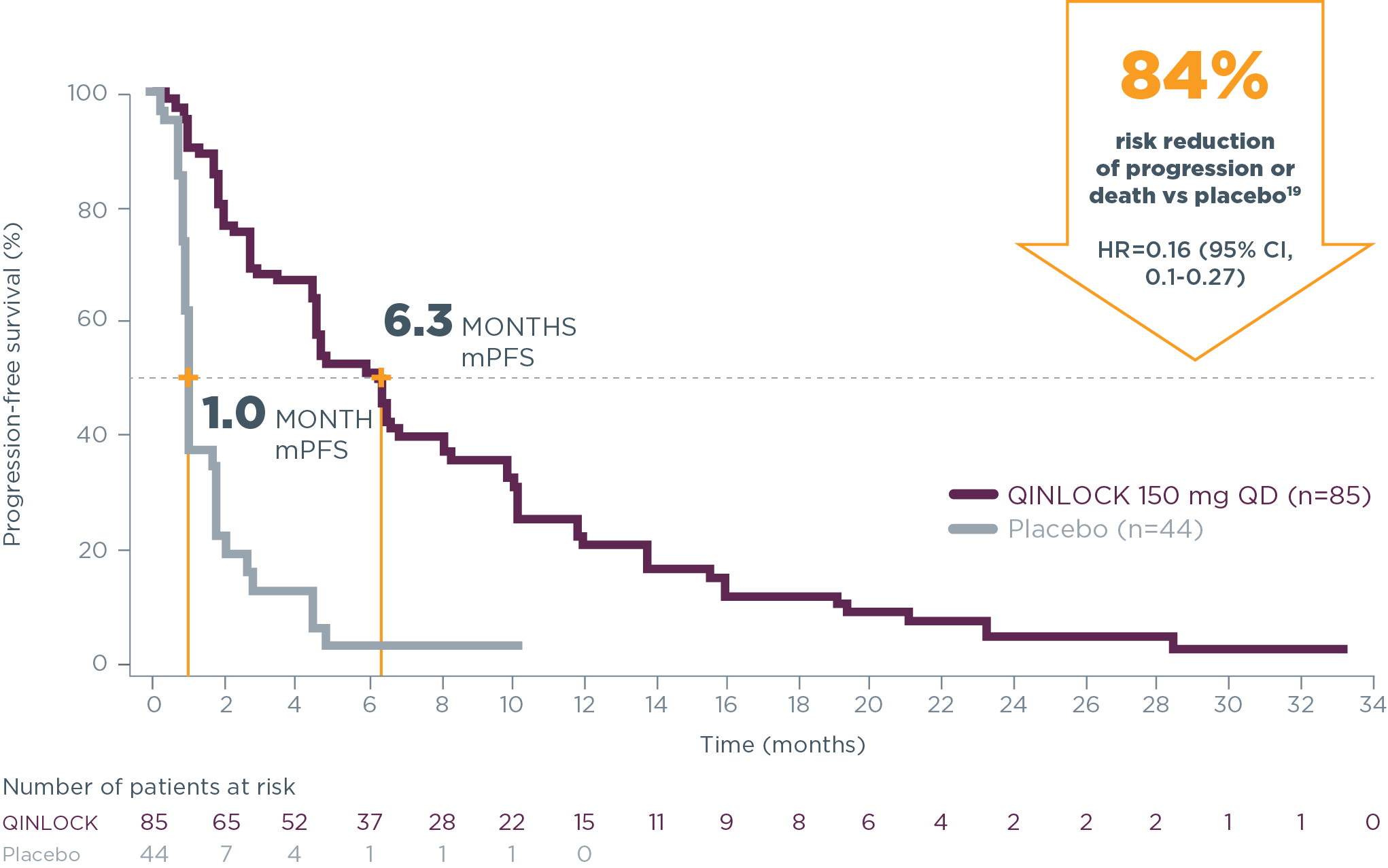
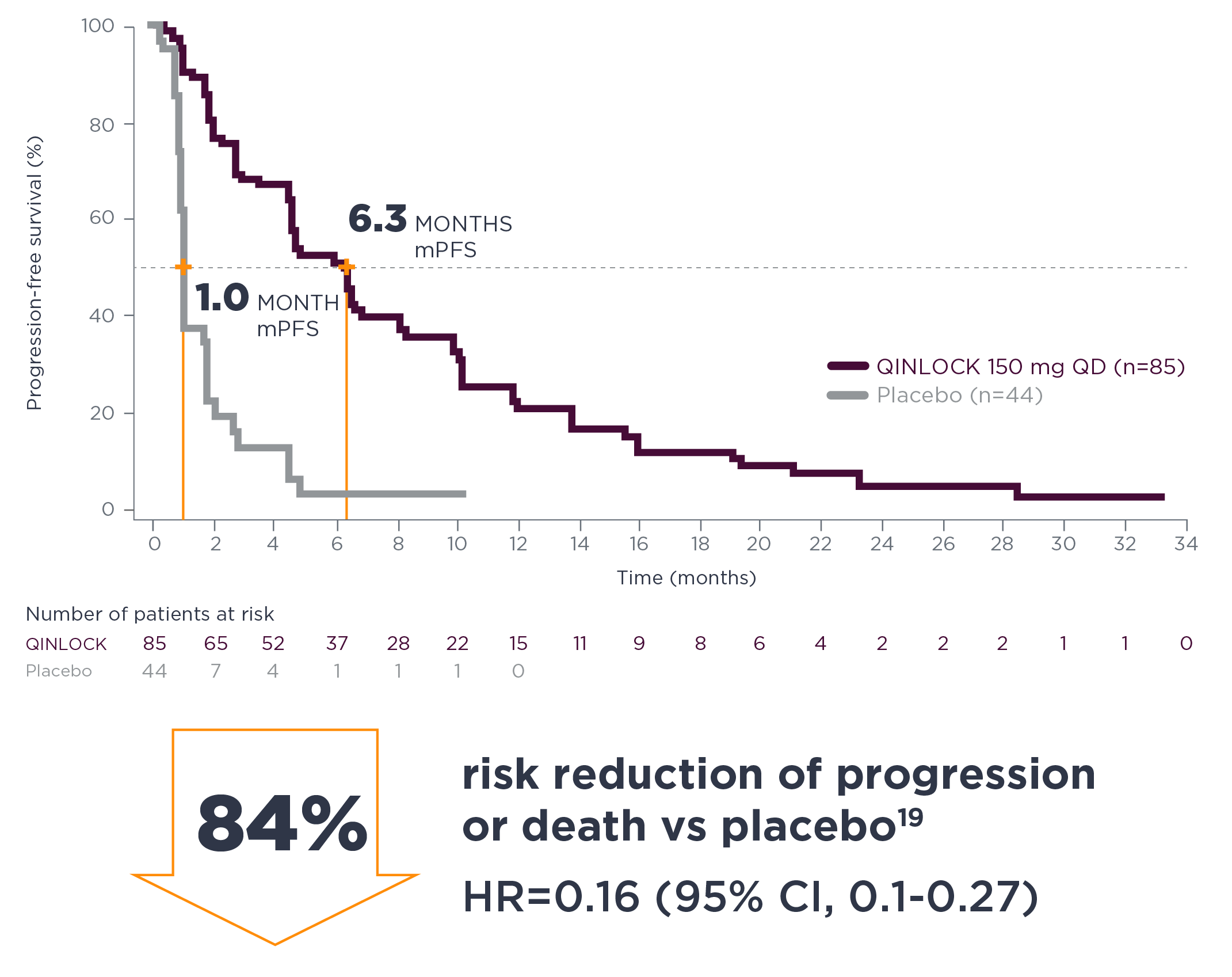
†The follow-up analysis was conducted approximately 19 months from the data cutoff in the primary analysis and was not powered to show statistical significance.4
Qinlock was studied in a global, Phase 3 study in ≥4th‑line GIST2
The INVICTUS study was a global, multicenter, randomized, double‑blind, placebo‑controlled Phase 3 trial (N=129).2
The patient population of INVICTUS was the most heavily pre-treated cohort ever studied in a Phase 3, randomized, 4th‑line GIST setting.2,5
63% of patients had received 3 prior therapies
37% of patients had received ≥4 prior therapies, and some as many as 7

Explore how QINLOCK was studied
More about INVICTUSCI=confidence interval; GIST=gastrointestinal stromal tumor; HR=hazard ratio; MOA=mechanism of action; mPFS=median progression-free survival; NCCN=National Comprehensive Cancer Network®; PFS=progression‑free survival; TKI=tyrosine kinase inhibitor.
References: 1. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastrointestinal Stromal Tumors (GIST) V.1.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed November 6, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 2. Qinlock [package insert]. Waltham, MA: Deciphera Pharmaceuticals, Inc; 2023. 3. Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC‑2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug‑resistant KIT and PDGFRA variants. Cancer Cell. 2019;35(5):738‑751. 4. von Mehren M, Heinrich M, George S, et al. Ripretinib as ≥4th‑line treatment in patients with advanced gastrointestinal stromal tumour (GIST): Long-term update from the phase 3 INVICTUS study. Poster presented at: 2021 European Society for Medical Oncology Virtual Meeting; September 16‑21, 2021. 5. Blay JY, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(7):923-934.